The Food and Drug Administration (FDA) yesterday announced the recall of three types of drugs over excessive impurities or failing a dissolution test, including a diabetes drug with a 100 percent share of the local market.
The three types of drugs are three batches of 100mg Januvia F.C. tablets (佳糖維100毫克膜衣錠), a batch of 30mg Lexinping capsules “Tai Yu” (台裕樂心平膠囊30毫克) and a batch of 10mg Sermion tablets (適脈旺糖衣錠10毫克).
The Januvia film-coated tablets, the main ingredient of which is sitagliptin (as monohydrate phosphate salt), is prescribed to adults with type 2 diabetes to improve blood sugar control.
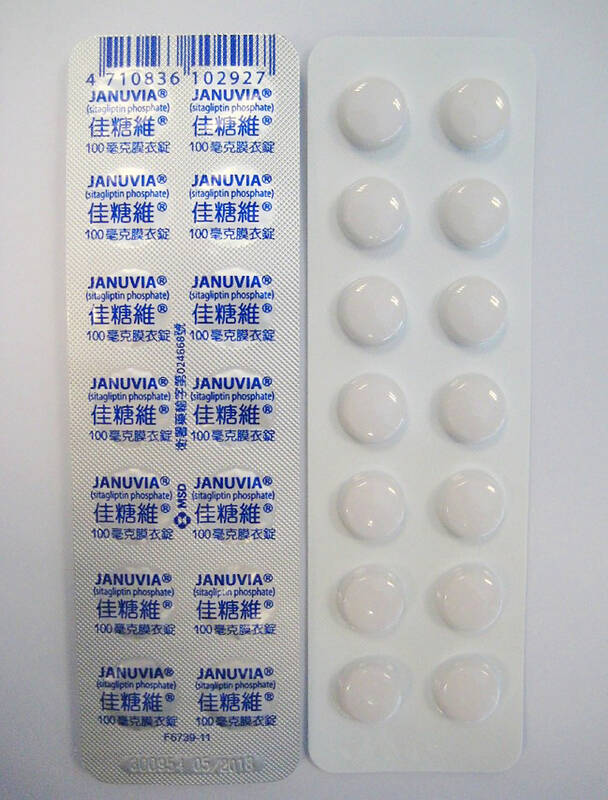
Photo copied by Wu Liang-yi, Taipei Times
Its drug permit holder, Merck Group Taiwan, had notified the FDA about finding excessive levels of impurities.
The three batches — U011910, U015917 and U018641 — would be recalled by Jan. 21, the FDA said.
Although the drug has a 100 percent market share, with more than 27 million tablets used annually, only about 1.2 million tablets are being recalled, said Hung Kuo-teng (洪國登), head of the FDA’s Medicinal Products Division.
There are other drugs with different ingredients that diabetes patients can use as replacement, Huang added.
Lexinping is mainly used to treat depression. The manufacturer decided to recall batch WE1304, comprising about 16,000 capsules, after samples taken from the batch were found to have a dissolution rate that failed to meet the specifications, which could affect the efficacy of the drug.
Hung said Lexinping capsules have only about a 3 percent market share, with about 820,000 capsules used per year, so there should not be a shortage of medication due to the recall, adding that the recall would run through Jan. 14.
Sermion tablets are mainly used to improve peripheral blood circulation. Its manufacturer, Viatris Pharmaceutical, detected impurities that failed to meet the specifications while conducting a stability test, and is recalling batch DN2443, which comprises about 2.7 million tablets.
Hung said that about 6.3 million Sermion tablets are used in the nation per year, translating into a market share of about 60 percent, but as there are alternative drugs, the recall would not cause a shortage of medication for patients.
Additional reporting by CNA

Global bodies should stop excluding Taiwan for political reasons, President William Lai (賴清德) told Pope Francis in a letter, adding that he agrees war has no winners. The Vatican is one of only 12 countries to retain formal diplomatic ties with Taiwan, and Taipei has watched with concern efforts by Beijing and the Holy See to improve ties. In October, the Vatican and China extended an accord on the appointment of Catholic bishops in China for four years, pointing to a new level of trust between the two parties. Lai, writing to the pope in response to the pontiff’s message on Jan. 1’s

A Vietnamese migrant worker on Thursday won the NT$12 million (US$383,590) jackpot on a scratch-off lottery ticket she bought from a lottery shop in Changhua County’s Puyan Township (埔鹽), Taiwan Lottery Co said yesterday. The lottery winner, who is in her 30s and married, said she would continue to work in Taiwan and send her winnings to her family in Vietnam to improve their life. More Taiwanese and migrant workers have flocked to the lottery shop on Sec 2 of Jhangshuei Road (彰水路) to share in the luck. The shop owner, surnamed Chen (陳), said that his shop has been open for just

HOLIDAY EXERCISE: National forest recreation areas from north to south offer travelers a wide choice of sights to connect with nature and enjoy its benefits Hiking is a good way to improve one’s health, the Forestry and Nature Conservation Agency said, as it released a list of national forest recreation areas that travelers can visit during the Lunar New Year holiday. Taking a green shower of phytoncides in the woods could boost one’s immunity system and metabolism, agency Director-General Lin Hwa-ching (林華慶) cited a Japanese study as saying. For people visiting northern Taiwan, Lin recommended the Dongyanshan National Forest Recreation Area in Taoyuan’s Fusing District (復興). Once an important plantation in the north, Dongyanshan (東眼山) has a number of historic monuments, he said. The area is broadly covered by

Tainan’s initiative to recruit digital nomads has resulted in several German, US and Vietnamese nationals applying to live and work in the city, the Tainan Research, Development and Evaluation Commission said yesterday. That marked the city as the first in the nation to attract digital nomads, following the launch of the program last month, it said. Although all applicants so far have used work visas or tourism visas instead of the special digital nomad permit from the Ministry of Foreign Affairs, the city government believes that the latter would be needed eventually, the commission said. The digital nomads recruited by Tainan would work