The Food and Drug Administration (FDA) yesterday said that 420 batches of 23 ranitidine drug products that allegedly contain the potentially carcinogenic n-nitrosodimethylamine (NDMA) must be recalled by Nov. 18.
The impurity was not intentionally added to the drugs by the manufacturers, but was found in the active pharmaceutical ingredients, the FDA said, adding that the source of the impurity remained unclear and was being investigated in several countries.
As part of normal monitoring of international drug safety information last month, the FDA discovered that some ranitidine drugs — used to treat and prevent ulcers in the stomach and intestines — were being voluntary recalled because NDMA was detected.

Photo courtesy of the Food and Drug
As NDMA is classified as a likely human carcinogen, it launched an inspection to test ranitidine products from multiple domestic manufacturers, the FDA said.
Thirty-eight permits for ranitidine products from 21 manufacturers have been approved by the FDA, and they were all asked to conduct their own laboratory testing to assess NDMA levels in their products and report the results to the agency within a month, it said.
As of Friday, the FDA had received reports for 15 products, or 287 batches, with those products approved to continue distribution and sales, FDA Medicinal Products Division section chief Hung Kuo-teng (洪國登) said.
The remaining 23 products must be recalled before Nov. 18, Hung said, adding that some of them contained NDMA exceeding the allowable limit, some did not turn in the report before the deadline and some were voluntarily recalled by the manufacturer.
A list of the products and batch numbers that have been recalled can be found on the FDA’s official Web site, www.fda.gov.tw, under the “stomach medicine abnormal events information” section on the Chinese-language main page.
Due to the volume of items affected by the recall, it is difficult to estimate totals for each product, Hung said.
Most of the recalled drugs were removed from shelves last month and there are alternative drugs that treat the same symptoms, so people do not need to worry, he said.
They should consult a doctor if they want to stop taking the drug or switch to an alternative, he said.
Additional reporting by staff writer, with CNA
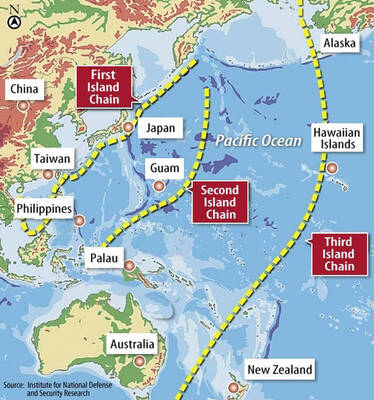
The US government has signed defense cooperation agreements with Japan and the Philippines to boost the deterrence capabilities of countries in the first island chain, a report by the National Security Bureau (NSB) showed. The main countries on the first island chain include the two nations and Taiwan. The bureau is to present the report at a meeting of the legislature’s Foreign Affairs and National Defense Committee tomorrow. The US military has deployed Typhon missile systems to Japan’s Yamaguchi Prefecture and Zambales province in the Philippines during their joint military exercises. It has also installed NMESIS anti-ship systems in Japan’s Okinawa

‘WIN-WIN’: The Philippines, and central and eastern European countries are important potential drone cooperation partners, Minister of Foreign Affairs Lin Chia-lung said Minister of Foreign Affairs Lin Chia-lung (林佳龍) in an interview published yesterday confirmed that there are joint ventures between Taiwan and Poland in the drone industry. Lin made the remark in an exclusive interview with the Chinese-language Liberty Times (the Taipei Times’ sister paper). The government-backed Taiwan Excellence Drone International Business Opportunities Alliance and the Polish Chamber of Unmanned Systems on Wednesday last week signed a memorandum of understanding in Poland to develop a “non-China” supply chain for drones and work together on key technologies. Asked if Taiwan prioritized Poland among central and eastern European countries in drone collaboration, Lin

BACK TO WORK? Prosecutors said they are considering filing an appeal, while the Hsinchu City Government said it has applied for Ann Kao’s reinstatement as mayor The High Court yesterday found suspended Hsinchu mayor Ann Kao (高虹安) not guilty of embezzling assistant fees, reducing her sentence to six months in prison commutable to a fine from seven years and four months. The verdict acquitted Kao of the corruption charge, but found her guilty of causing a public official to commit document forgery. The High Prosecutors’ Office said it is reviewing the ruling and considering whether to file an appeal. The Taipei District Court in July last year sentenced Kao to seven years and four months in prison, along with a four-year deprivation of civil rights, for contravening the Anti-Corruption

NO CONFIDENCE MOTION? The premier said that being toppled by the legislature for defending the Constitution would be a democratic badge of honor for him Premier Cho Jung-tai (卓榮泰) yesterday announced that the Cabinet would not countersign the amendments to the local revenue-sharing law passed by the Legislative Yuan last month. Cho said the decision not to countersign the amendments to the Act Governing the Allocation of Government Revenues and Expenditures (財政收支劃分法) was made in accordance with the Constitution. “The decision aims to safeguard our Constitution,” he said. The Constitution stipulates the president shall, in accordance with law, promulgate laws and issue mandates with the countersignature of the head of the Executive Yuan, or with the countersignatures of both the head of the Executive Yuan and ministers or