Vaccine maker Adimmune Corp (國光生技) has gained an approval from Indonesia’s health regulator to conduct phase 1 and 2 clinical trials for its experimental COVID-19 vaccine in the Southeast Asian country, as it aims to tap the Muslim-based market with halal certification, the company said yesterday.
The trials in Indonesia would be Adimmune’s first overseas human tests for its experimental COVID-19 vaccine, after the Ministry of Health and Welfare ordered it to redo its phase 1 trial in Taiwan in December last year to determine the optimal dosage.
Adimmune yesterday said that the Indonesian trials would determine the optimal dosage for its vaccine to generate adequate protection against COVID-19 variants and prove its efficacy.

Photo courtesy of Adimmune Corp
The company plans to recruit 240 healthy adults aged between 18 and 65 to participate in the phase 2 trials, spokesman Pan Fei (潘飛) told the Taipei Times yesterday.
Despite encountering a setback in Taiwan, Adimmune did not modify the formula of its COVID-19 vaccine, dubbed AdimrSC-2f, nor its recombinant protein manufacturing technology, Pan said.
Adimmune’s candidate was developed in spring last year, before the emergence of the SARS-CoV-2 Delta variant. However, the company said it had tested its candidate against the variant in animal tests earlier this year, and that it showed good potential.
Unlike most finished clinical trials, which provide participants with two doses of an experimental vaccine, Adimmune’s clinical trials in Indonesia would give participants three doses, Pan said, adding that the company’s phase 2 trial would require more time and results are expected to be released next year.
The company is to produce the experimental COVID-19 vaccine at its factory in Taiwan and export it to Indonesia for the human trials, in collaboration with the Faculty of Medicine and Health Sciences at Universitas Gadjah Mada, Pan said.
Adimmune said it expects to complete the phase 1 and 2 trials early next year, but it would not seek emergency use authorization (EUA) upon completion, Pan said.
Instead, the company plans to apply to the Indonesian regulator to conduct a phase 3 trial there, he said.
“Timing for applying for an EUA has passed, as there are many available COVID-19 vaccines on the market,” Pan said. “Besides, an EUA is temporary. We aim to conduct the clinical trials without missing any steps and apply for a marketing approval that is valid for a long time.”
If Adimmune gains marketing approval from Indonesia for its COVID-19 vaccines, there is a good chance that the company would be able to provide its vaccine to other Muslim markets, Pan said, adding that the company has obtained a halal certification with its COVID-19 vaccine candidate.
Indonesia has the world’s largest Muslim population, consisting of about 87 percent of its 270.6 million inhabitants.
As with Moderna Inc, Adimmune would consider combining its flu vaccine into COVID-19 vaccine if its candidate is approved, although that mixture would not be a focus for research and development at this time, Pan said.
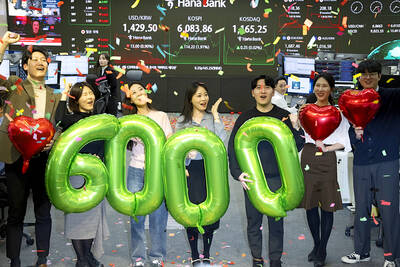
South Korea’s equity benchmark yesterday crossed a new milestone just a month after surpassing the once-unthinkable 5,000 mark as surging global memory demand powers the country’s biggest chipmakers. The KOSPI advanced as much as 2.6 percent to a record 6,123, with Samsung Electronics Co and SK Hynix Inc each gaining more than 2 percent. With the benchmark now up 45 percent this year, South Korea’s stock market capitalization has also moved past France’s, following last month’s overtaking of Germany’s. Long overlooked by foreign funds, despite being undervalued, South Korean stocks have now emerged as clear winners in the global market. The so-called “artificial intelligence

CONFUSION: Taiwan, Japan and other big exporters are cautiously monitoring the situation, while analysts said more Trump responses ate likely after his loss in court US trading partners in Asia started weighing fresh uncertainties yesterday after President Donald Trump vowed to impose a new tariff on imports, hours after the Supreme Court struck down many of the sweeping levies he used to launch a global trade war. The court’s ruling invalidated a number of tariffs that the Trump administration had imposed on Asian export powerhouses from China and South Korea to Japan and Taiwan, the world’s largest chip maker and a key player in tech supply chains. Within hours, Trump said he would impose a new 10 percent duty on US imports from all countries starting on

Chinese artificial intelligence (AI) start-up DeepSeek’s (深度求索) latest AI model, set to be released as soon as next week, was trained on Nvidia Corp’s most advanced AI chip, the Blackwell, a senior official of US President Donald Trump’s administration said on Monday, in what could represent a violation of US export controls. The US believes DeepSeek will remove the technical indicators that might reveal its use of American AI chips, the official said, adding that the Blackwells are likely clustered at its data center in Inner Mongolia, an autonomous region of China. The person declined to say how the US government received

Like many of us who are mindful of our plastic consumption, Beth Gardiner would take her own bags to the supermarket and be annoyed whenever she forgot to do so. Out without her refillable bottle, she would avoid buying bottled water. “Here I am, in my own little life, worrying about that and trying to use less plastic,” she says. Then she read an article in this newspaper, just over eight years ago, and discovered that fossil fuel companies had plowed more than US$180 billion into plastic plants in the US since 2010. “It was a kick in the teeth,” Gardiner