Vaccine maker Adimmune Corp (國光生技) plans to conduct phase I clinical trials of its COVID-19 candidate vaccine next month to assess its safety, the firm said yesterday.
Adimmune expects to receive approval from the Food and Drug Administration (FDA) later this month to start trials, as the review has been going smoothly so far, Adimmune spokesman Pan Fei (潘飛) told the Taipei Times by telephone.
The vaccine provider would cooperate with National Taiwan University Hospital (NTUH) for the trials, Pan said.
The company plans to enroll 60 to 80 healthy adult participants, who would be divided into several groups and receive vaccines at different doses, to see if the experimental vaccine is safe, Pan said.
“We will monitor if there are any severe side effects to the participants and if they produce coronavirus antibodies. Based on the results of phase I trials, we will select two dose levels that show the most promise to continue with phase II clinical trials,” Pan said.
Although Minister of Health and Welfare Chen Shih-chung (陳時中) last week said that the government would allow biotechnology firms to skip phase III clinical trials of their vaccine candidates to accelerate vaccine development, Adimmune would not eschew the final phase of human trials, Pan said.
“We are considering combining phase II and phase III trials into a phase II/III design to save time and reduce the total number of test participants,” he said.
The firm still needs to discuss with the regulator the protocol for a phase II/III trial, including clinical endpoints and the number of enrolled participants, he added.
Adimmune, the nation’s largest flu vaccine provider, is a frontrunner in the local race to develop a COVID-19 vaccine, as it was the first to apply to the FDA to conduct human trials.
Its vaccine candidate, developed using the novel coronavirus’ genetic sequence and the firm’s recombinant protein technology, in an animal test in April caused laboratory mice to produce antibodies that could inhibit the growth of the coronavirus, company data showed.
The firm’s achievements have been rewarded by investors, with its stock price rising from NT$36.35 on May 6 — when it announced the results of its animal test — to NT$75.6 at the close of Taipei trading yesterday.
However, the company did not take advantage of its high stock price to raise capital, as it did not issue new common shares, but took out a NT$4.2 billion (US$142 million) syndicated loan from nine local banks to fuel research, new factories and international development.
“Taking the syndicated loan did not increase our financial burden thanks to lower interest rates while we did not want to expand the number of shares in the short term due to concerns over stock dilution,” Pan said.
Last week, Medigen Vaccine Biologics Corp (高端疫苗) applied to the FDA to run phase I human trials for its spike protein-based vaccine candidate, becoming second in the local race to produce a COVID-19 vaccine, company data showed.
Separately yesterday, Golden Biotechnology Corp (GBC, 國鼎生物科技) announced that it has gained approval from the US Food and Drug Administration to conduct phase II human trials for its small molecule drug, Antroquinonol (Hocena), as COVID-19 treatment, it said in a filing with the Taipei Exchange.
The drug, originally developed to treat pancreatic cancer, has proven safe in its phase I human trial in the US, the firm said.

GREAT SUCCESS: Republican Senator Todd Young expressed surprise at Trump’s comments and said he expects the administration to keep the program running US lawmakers who helped secure billions of dollars in subsidies for domestic semiconductor manufacturing rejected US President Donald Trump’s call to revoke the 2022 CHIPS and Science Act, signaling that any repeal effort in the US Congress would fall short. US Senate Minority Leader Chuck Schumer, who negotiated the law, on Wednesday said that Trump’s demand would fail, while a top Republican proponent, US Senator Todd Young, expressed surprise at the president’s comments and said he expects the administration to keep the program running. The CHIPS Act is “essential for America leading the world in tech, leading the world in AI [artificial
DOMESTIC SUPPLY: The probe comes as Donald Trump has called for the repeal of the US$52.7 billion CHIPS and Science Act, which the US Congress passed in 2022 The Office of the US Trade Representative is to hold a hearing tomorrow into older Chinese-made “legacy” semiconductors that could heap more US tariffs on chips from China that power everyday goods from cars to washing machines to telecoms equipment. The probe, which began during former US president Joe Biden’s tenure in December last year, aims to protect US and other semiconductor producers from China’s massive state-driven buildup of domestic chip supply. A 50 percent US tariff on Chinese semiconductors began on Jan. 1. Legacy chips use older manufacturing processes introduced more than a decade ago and are often far simpler than

Hon Hai Precision Industry Co (鴻海精密) yesterday said that its research institute has launched its first advanced artificial intelligence (AI) large language model (LLM) using traditional Chinese, with technology assistance from Nvidia Corp. Hon Hai, also known as Foxconn Technology Group (富士康科技集團), said the LLM, FoxBrain, is expected to improve its data analysis capabilities for smart manufacturing, and electric vehicle and smart city development. An LLM is a type of AI trained on vast amounts of text data and uses deep learning techniques, particularly neural networks, to process and generate language. They are essential for building and improving AI-powered servers. Nvidia provided assistance
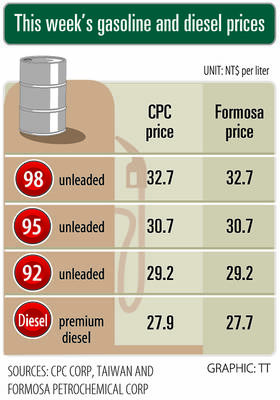
Gasoline and diesel prices this week are to decrease NT$0.5 and NT$1 per liter respectively as international crude prices continued to fall last week, CPC Corp, Taiwan (CPC, 台灣中油) and Formosa Petrochemical Corp (台塑石化) said yesterday. Effective today, gasoline prices at CPC and Formosa stations are to decrease to NT$29.2, NT$30.7 and NT$32.7 per liter for 92, 95 and 98-octane unleaded gasoline respectively, while premium diesel is to cost NT$27.9 per liter at CPC stations and NT$27.7 at Formosa pumps, the companies said in separate statements. Global crude oil prices dropped last week after the eight OPEC+ members said they would