Medigen Vaccine Biologics Corp (高端疫苗) has obtained certification to market its COVID-19 reagent, which uses a polymerase chain reaction (PCR) technique, in the eurozone.
“The approval gave us access to the European market, but as our testing kits are produced in Taiwan, we need to gain marketing approval from the Taiwan Food and Drug Administration before exporting those products,” spokesman Leo Lee (李思賢) told the Taipei Times by telephone on Monday.
The crisis could accelerate the agency’s review, compared with at least one year in the past, Lee said.
Given the shortage of coronavirus testing kits, foreign buyers have shown an interest in the firm’s products, he said, adding that Medigen’s local plant has produced some kits.
For an in vitro diagnostic device, the firm spent one month gaining CE marking certification, which is required for products sold in the European Economic Area, under an emergency-use program initiated by the European Commission.
Medigen’s diagnostic reagent was the latest to obtain the certification, after products manufactured by GeneReach Biotechnology Corp (瑞基海洋) and General Biologicals Corp (普生).
Like the other companies’ products, Medigen’s reagent gives results in about 80 minutes, with an accuracy rate of 95 percent, Lee said.
As some people infected with COVID-19 have shown false negatives, the public has questioned the accuracy of the testing kits that are available.
“The false negatives are not the result of a failure on the part of the testing kits, as most medical devices are sensitive and accurate. It is more likely attributable to how the samples are collected,” Lee said.
If the viral load on a throat swab is too low, the result cannot be positive, so it is important to conduct the tests several times, he said.
Medigen is not concerned that competition in the coronavirus reagent business could heat up too much as foreign companies develop antibody testing kits, Lee said, adding that PCR testing kits identify the coronavirus, while the antibody blood tests determine whether a person’s immune system has developed antibodies against the virus.
“The former determines if those tested would infect others, while the latter determines if the person has recovered and can come out of isolation,” Lee said.
The two products are used in a complementary way, he added.
Separately, active pharmaceutical ingredient maker Formosa Laboratories Inc (台耀化學) on Monday said that it collaborated with National Yang-Ming University in Taipei, National Chiao Tung University in Hsinchu and Taipei Veterans General Hospital in synthesizing 168g of remdesivir, a new antiviral drug by US-based Gilead Sciences Inc that targets infectious diseases such as Ebola and SARS, and that has shown some effectiveness against COVID-19.
Formosa Laboratories told local media that it has spent NT$4 million (US$133,174) on capacity to produce remdesivir.
The firm has also funded research on the production of favipiravir, the main ingredient in the influenza drug Avigan, which was developed by Fujifilm Holdings Corp in Japan and is being tested as a potential treatment for COVID-19.
Additional reporting by staff writer

Hon Hai Precision Industry Co (鴻海精密) yesterday said that its research institute has launched its first advanced artificial intelligence (AI) large language model (LLM) using traditional Chinese, with technology assistance from Nvidia Corp. Hon Hai, also known as Foxconn Technology Group (富士康科技集團), said the LLM, FoxBrain, is expected to improve its data analysis capabilities for smart manufacturing, and electric vehicle and smart city development. An LLM is a type of AI trained on vast amounts of text data and uses deep learning techniques, particularly neural networks, to process and generate language. They are essential for building and improving AI-powered servers. Nvidia provided assistance

GREAT SUCCESS: Republican Senator Todd Young expressed surprise at Trump’s comments and said he expects the administration to keep the program running US lawmakers who helped secure billions of dollars in subsidies for domestic semiconductor manufacturing rejected US President Donald Trump’s call to revoke the 2022 CHIPS and Science Act, signaling that any repeal effort in the US Congress would fall short. US Senate Minority Leader Chuck Schumer, who negotiated the law, on Wednesday said that Trump’s demand would fail, while a top Republican proponent, US Senator Todd Young, expressed surprise at the president’s comments and said he expects the administration to keep the program running. The CHIPS Act is “essential for America leading the world in tech, leading the world in AI [artificial
DOMESTIC SUPPLY: The probe comes as Donald Trump has called for the repeal of the US$52.7 billion CHIPS and Science Act, which the US Congress passed in 2022 The Office of the US Trade Representative is to hold a hearing tomorrow into older Chinese-made “legacy” semiconductors that could heap more US tariffs on chips from China that power everyday goods from cars to washing machines to telecoms equipment. The probe, which began during former US president Joe Biden’s tenure in December last year, aims to protect US and other semiconductor producers from China’s massive state-driven buildup of domestic chip supply. A 50 percent US tariff on Chinese semiconductors began on Jan. 1. Legacy chips use older manufacturing processes introduced more than a decade ago and are often far simpler than
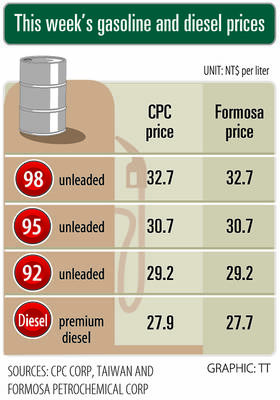
Gasoline and diesel prices this week are to decrease NT$0.5 and NT$1 per liter respectively as international crude prices continued to fall last week, CPC Corp, Taiwan (CPC, 台灣中油) and Formosa Petrochemical Corp (台塑石化) said yesterday. Effective today, gasoline prices at CPC and Formosa stations are to decrease to NT$29.2, NT$30.7 and NT$32.7 per liter for 92, 95 and 98-octane unleaded gasoline respectively, while premium diesel is to cost NT$27.9 per liter at CPC stations and NT$27.7 at Formosa pumps, the companies said in separate statements. Global crude oil prices dropped last week after the eight OPEC+ members said they would