The Food and Drug Administration (FDA) is concealing pharmaceutical companies’ violations of the law that have made members of the public “guinea pigs,” a health insurance watchdog group said yesterday.
Good Manufacturing Practice (GMP) inspections conducted by the FDA earlier this year of pharmaceutical manufacturers found that many drugs had been manufactured in a way that failed to comply with what had been described in their marketing authorizations, Democratic Progressive Party Legislator Chen Chieh-ju (陳節如) said.
The inconsistencies are in violation of the Pharmaceutical Affairs Act (藥事法), the Pharmaceutical Good Manufacturing Practice Regulations (藥物優良製造準則) and the Regulations for Registration of Medicinal Products (藥品查驗登記審查準則), and the firms and the FDA have placed patients at risk due to inadequate safety and quality of the drugs produced, Chen said in a joint statement issued with the National Health Insurance Civilian Surveillance Alliance.
The alleged changes, without seeking the approval of the regulator, have been made to package inserts, packaging and the excipient used, the group said.
However, the most dangerous of the violations has been the failure to provide bioequivalence (BE) test reports, or reports on clinical trials and whether, by monitoring the differences in blood levels, a potential to-be-marketed generic drug product is bioequivalent to a reference brand-name pharmaceutical, it added.
“As of this March, the FDA found at least 3,841 items that failed to comply with the regulations. We have no idea how many of these drugs did not have BE reports, since a request to the FDA for the relevant information has been denied by the authority, which cited the Personal Information Protection Act,” Huang Sue-ying (黃淑英), convener of the alliance, told a press conference at the legislature in Taipei.
The FDA has not asked firms producing these substandard products to suspend manufacturing nor have the products been withdrawn from the market. The list of products and the relevant information has not been made public, which means patients and healthcare providers cannot avoid using these drugs, she added.
What has enraged the group is how the drug companies’ actions were exposed. The FDA made an announcement on March 28 saying that for drugs without a BE report, but which have been on the market for at least five years and thereby have a long-term clinical experience, “their clinical experience can substitute for the required BE test if no serious adverse effects or therapeutic inequivalence have been reported.”
“This is no different to exploiting patients, allowing them to become the manufacturers’ guinea pigs at the NHI’s expense,” alliance spokeswoman Eva Teng (滕西華) said.
What is more, drugs that were manufactured and marketed after Jan. 1, 2009, and which therefore do not meet the five-year requirement, have been granted a two-year probationary period to complete BE tests without a suspension of sales of the drug during the testing period, Huang said.
“The BE test reports done by the these manufacturers are only subject to random spot-checks by the FDA,” Huang said.
In response, FDA official Tzou Meir-chyun (鄒玫君), who was also present at the press conference, said all pharmaceutical products currently on the market have been approved by the FDA, with BE tests conducteds before being approved.
“They are all post-approval changes. As post-approval changes can be, according to international standards, categorized into major and minor changes, it is only when major changes take place that a further risk assessment is needed,” Tzou said.
The FDA released a statement later in the day saying that a risk assessment meeting evaluating the drugs that underwent major changes — about 5 percent of the drugs where post-approval changes have been made — would be held within the next month.
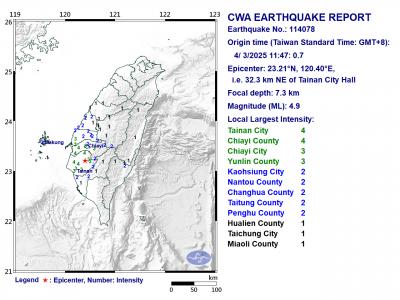
A magnitude 4.9 earthquake struck off Tainan at 11:47am today, the Central Weather Administration (CWA) said. The hypocenter was 32.3km northeast of Tainan City Hall at a depth of 7.3km, CWA data showed. The intensity of the quake, which gauges the actual effect of a seismic event, measured 4 in Tainan and Chiayi County on Taiwan's seven-tier intensity scale, the data showed. The quake had an intensity of 3 in Chiayi City and County, and Yunlin County, while it was measured as 2 in Kaohsiung, Nantou County, Changhua County, Taitung County and offshore Penghu County, the data showed. There were no immediate reports of

The Chinese Nationalist Party (KMT) is maintaining close ties with Beijing, the Democratic Progressive Party (DPP) said yesterday, hours after a new round of Chinese military drills in the Taiwan Strait began. Political parties in a democracy have a responsibility to be loyal to the nation and defend its sovereignty, DPP spokesman Justin Wu (吳崢) told a news conference in Taipei. His comments came hours after Beijing announced via Chinese state media that the Chinese People’s Liberation Army’s Eastern Theater Command was holding large-scale drills simulating a multi-pronged attack on Taiwan. Contrary to the KMT’s claims that it is staunchly anti-communist, KMT Deputy

RESPONSE: The government would investigate incidents of Taiwanese entertainers in China promoting CCP propaganda online in contravention of the law, the source said Taiwanese entertainers living in China who are found to have contravened cross-strait regulations or collaborated with the Chinese Communist Party (CCP) could be subject to fines, a source said on Sunday. Several Taiwanese entertainers have posted on the social media platform Sina Weibo saying that Taiwan “must be returned” to China, and sharing news articles from Chinese state media. In response, the Mainland Affairs Council (MAC) has asked the Ministry of Culture to investigate whether the entertainers had contravened any laws, and asked for them to be questioned upon their return to Taiwan, an official familiar with the matter said. To curb repeated

Taiwan has recorded its first fatal case of Coxsackie B5 enterovirus in 10 years after a one-year-old boy from southern Taiwan died from complications early last month, the Centers for Disease Control (CDC) said yesterday. CDC spokesman Lo Yi-chun (羅一鈞) told a news conference that the child initially developed a fever and respiratory symptoms before experiencing seizures and loss of consciousness. The boy was diagnosed with acute encephalitis and admitted to intensive care, but his condition deteriorated rapidly, and he passed away on the sixth day of illness, Lo said. This also marks Taiwan’s third enterovirus-related death this year and the first severe