The process through which Taichung County-based biotech company Adimmune Corp came to manufacture its A(H1N1) influenza vaccine was flawed and paved the way for safety concerns that have surrounded the vaccine, a US expert on infectious diseases told the Taipei Times this week.
Ho Mei-shang (何美鄉), a research fellow at Academia Sinica’s Institute of Biomedical Sciences and former director of biotechnology research at Adimmune, disputes this view, however, saying in an interview on Tuesday that the vaccine is safe and that there was nothing wrong with the process.
The Centers for Disease Control (CDC) announced on July 13 that Adimmune, the sole bidder, had won a government contract to produce 5 million doses of the (A)H1N1 vaccine, at NT$199 per dose, for delivery by the end of October. By early this month, Adimmune had provided 6.95 million doses, while Switzerland-based Novartis had provided 2.02 million (at about NT$400 per dose), out of the government’s purchases of 10 million and 5 million doses respectively.
The government has spent NT$3 billion (US$94.4 million) purchasing vaccines from Adimmune.
The problems begin when looking at the chain of events that led to the awarding of the contract, the US expert said, speaking on condition of anonymity because of the sensitive nature of her position.
As early as May 5, the CDC said that Adimmune, in cooperation with the National Health Research Institutes, would manufacture a domestic influenza vaccine. This was more than a month before a WHO survey of global flu vaccine production capacity on July 7 concluded that countries such as Taiwan that had not placed early orders for the vaccine would not get any vaccine after November.
Adimmune’s chairman is Steve Chan (詹啟賢), deputy secretary-general of the Chinese Nationalist Party (KMT). Chan was also the deputy executive director of President Ma Ying-jeou’s (馬英九) presidential campaign.
For decades a producer of animal vaccines, Adimmune is the only firm in Taiwan with the capacity to produce human vaccines.
Prior to the A(H1N1) contract, it had never produced human vaccines on its own. All it did, the source said, was “package” vaccines made by other, mostly Japanese, companies.
Ho maintained, however, that through close cooperation with Japanese and Dutch vaccine makers, Adimmune “leapfrogged” and obtained the know-how to make human vaccines.
Most vaccine manufacturers, including GSK and Novartis, have decades of experience producing seasonal flu vaccines. While H1N1 is only a variant of the seasonal flu, major manufacturers usually obtain licensure from the US Food and Drug Administration (FDA) to expedite the process based on regulations regarding “strain change.”
Regardless, manufacturers providing H1N1 vaccines to the US have undergone clinical trials, most of which are sponsored by the National Institutes of Health (NIH).
EXPEDITED PROCESS
Adimmune, which had no experience producing the vaccine, should never have been allowed to enter an “expedited process,” the source said.
Adimmune only conducted a small-scale clinical trial with less than 500 subjects, far less than the 5,000 to 6,000 that are usually required for new vaccines.
Ho said that as the vaccine is based on the seasonal flu vaccine, it is not a new vaccine and therefore there was “no point” in conducting more rigorous testing.
The source, however, said that as Adimmune had never produced the seasonal flu vaccine, the Department of Health (DOH) should have demanded the company conduct more clinical trials.
“This is the scientific foundation to claim that a vaccine is ‘safe,’” the source said. “You can’t cut corners on this. Science requires replicability.”
In response, Ho said that, “lack of experience does not jeopardize quality. It just means more costs.”
Adimmune’s chairman has said that clinical trials are a “redundant procedure for bureaucrats,” adding that most Western countries had shortened them to speed up vaccinations.
In other words, despite its lack of experience, the company felt confident it could produce a safe product with few trials, while more experienced manufacturers that also entered an “expedited process” proceeded more cautiously — GSK did 6,340 tests, Novartis did 4,768 and the NIH conducted 3,951, data shows.
(The Novartis version of the vaccine sold to Taiwan is a H5N1 markup with an adjuvant, which was purchased via a special procurement as the adjuvant has yet to be approved in Taiwan.)
WHO guidelines for vaccine manufacturing show that three demo batches of a vaccine, as well as pre-approval inspection, must be completed before a vaccine can be released.
The source said Adimmune finished the clinical study on Oct. 21 and obtained the licensure on Nov. 12. The nationwide vaccine program started on Nov. 15.
“According to the timeline, Adimmune couldn’t have made it in three weeks,” the source said. “This means they mass produced before an approval [license] was granted,” the source said.
Ho confirmed this was the case.
“Adimmune was taking a chance,” Ho said, because if it failed to obtain approval, it would have “to discard all batches that had been produced beforehand.”
“I was among the very few top management team and I was adamant about going ahead to produce the vaccine as at that time, there appeared to [be] no other opportunity to secure H1N1 vaccines for ... Taiwan,” Ho said.
“My intention at the time was that we, the Taiwanese people ... would lose more if there was no H1N1 vaccine at all. Thus, with a certain degree of confidence in what Adimmune could achieve, the risk of failure was deemed slim and we went ahead,” Ho said.
“There was no ethical issue. It was all about regulation and it was OK as long as approval was obtained eventually,” she said.
“Because the approval was based on the inspection and documentation of consistency of three consecutive [vaccine] batches — even though the paperwork was completed later — it was retroactively applicable,” she said.
On Jan. 7, the DOH said autopsies showed that six of 17 deaths possibly related to the vaccine were due to other causes, with investigations continuing on the other 11.
Ho confirmed that all cases involved individuals who had received the Adimmune vaccine, adding that this didn’t mean the vaccine was less safe than Novartis’, as the majority of vaccines administered here were Adimmune’s.
The source also saw problems with the DOH.
ETHICS
“Again and again, DOH officials have sided with Adimmune, something I have never seen in the US,” the source said. “If the commissioner of the [US] FDA, the head of the [US] CDC or the NIH endorsed a certain brand, they not only would be in clear violation of ethics codes, they would be committing political suicide.”
When a legislator asked to monitor the egg production process (influenza vaccines are produced in fertilized chicken eggs), Adimmune said “it’s a commercial secret,” the source said. “Later, when the CDC head [Steve Kuo, 郭旭崧] was asked how the sterility of the eggs was assured, his answer was: ‘Adimmune examined it.’”
“Was there no DOH oversight?” the source asked.
Ho maintains that from the very beginning, the DOH kept a professional distance from Adimmune. There was no dialogue between Adimmune and the CDC, which was responsible for buying the vaccine, she said.
Despite Adimmune’s requests, the DOH also hasn’t allowed it to release clinical test results.
The reason, Ho said, is that “technically speaking, the clinical trial has not been finished.”
The contract stipulates that until then, no information can be disclosed.
“The DOH adamantly opposes disclosure,” Ho said. “That makes me think something is fishy.”
For the source, the vaccine mess is another example of the gambling attitude of the Ma administration, conflict of interest and lack of transparency.
“As a federal government employee with experience in issuing contracts, and as a medical professional, I would say that if the same process occurred in the US, not only would it [make] the headlines in the Washington Post, but criminal investigations would follow,” she said.
For Ho, the problem was poor government communication and media speculation. Chan’s role as chairman also made it easier to politicize the matter, she said.
“Adimmune is more than a private company — it does public health,” she said. “The firm could have gone bankrupt, but everybody worked hard to make it happen in time and provide Taiwan with a vaccine production facility.”
Asked if she’d taken the vaccine, Ho said, “Yes, — Adimmune’s.”

DEFENSE: The National Security Bureau promised to expand communication and intelligence cooperation with global partners and enhance its strategic analytical skills China has not only increased military exercises and “gray zone” tactics against Taiwan this year, but also continues to recruit military personnel for espionage, the National Security Bureau (NSB) said yesterday in a report to the Legislative Yuan. The bureau submitted the report ahead of NSB Director-General Tsai Ming-yen’s (蔡明彥) appearance before the Foreign and National Defense Committee today. Last year, the Chinese People’s Liberation Army (PLA) conducted “Joint Sword-2024A and B” military exercises targeting Taiwan and carried out 40 combat readiness patrols, the bureau said. In addition, Chinese military aircraft entered Taiwan’s airspace 3,070 times last year, up about
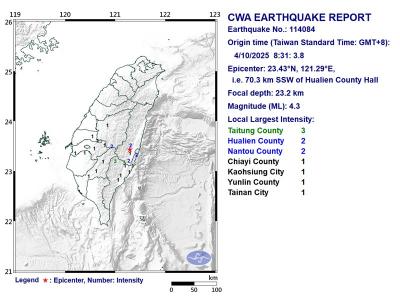
A magnitude 4.3 earthquake struck eastern Taiwan's Hualien County at 8:31am today, according to the Central Weather Administration (CWA). The epicenter of the temblor was located in Hualien County, about 70.3 kilometers south southwest of Hualien County Hall, at a depth of 23.2km, according to the administration. There were no immediate reports of damage resulting from the quake. The earthquake's intensity, which gauges the actual effect of a temblor, was highest in Taitung County, where it measured 3 on Taiwan's 7-tier intensity scale. The quake also measured an intensity of 2 in Hualien and Nantou counties, the CWA said.

The Overseas Community Affairs Council (OCAC) yesterday announced a fundraising campaign to support survivors of the magnitude 7.7 earthquake that struck Myanmar on March 28, with two prayer events scheduled in Taipei and Taichung later this week. “While initial rescue operations have concluded [in Myanmar], many survivors are now facing increasingly difficult living conditions,” OCAC Minister Hsu Chia-ching (徐佳青) told a news conference in Taipei. The fundraising campaign, which runs through May 31, is focused on supporting the reconstruction of damaged overseas compatriot schools, assisting students from Myanmar in Taiwan, and providing essential items, such as drinking water, food and medical supplies,

New Party Deputy Secretary-General You Chih-pin (游智彬) this morning went to the National Immigration Agency (NIA) to “turn himself in” after being notified that he had failed to provide proof of having renounced his Chinese household registration. He was one of more than 10,000 naturalized Taiwanese citizens from China who were informed by the NIA that their Taiwanese citizenship might be revoked if they fail to provide the proof in three months, people familiar with the matter said. You said he has proof that he had renounced his Chinese household registration and demanded the NIA provide proof that he still had Chinese