Readers of a squeamish disposition, look away now. The following article has vivid descriptions of stomach-churning experiments, freakish deformity and sex. Lots of sex, often done very badly. You really might be better off trying Sudoku.
"Human babies grown in a laboratory," a front-page story in a British newspaper screamed earlier this month. The story, of course, was wrong. It was unfertilized human egg cells that had been produced -- but could the overexcited headline be a sign of things to come?
In their efforts to tackle inherited diseases and help infertile couples, scientists across the world are developing techniques and technology that ape the most basic -- and morally complicated -- of biological functions: human reproduction. Taken together, the work poses some troubling questions.
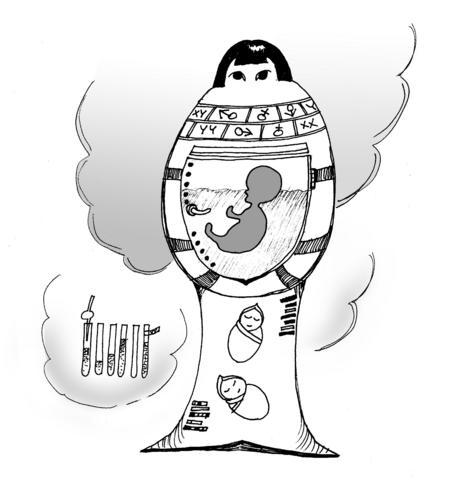
ILLUSTRATION: TANIA
In the most recent research, the scientists claim to have grown eggs using stem cells scraped from anonymous human tissue. Others are trying to do the same with sperm. How long before they succeed? And could the two be combined to produce a synthetic embryo? No serious scientist advocates such a move, but, as the parallel field of human cloning demonstrates, not everyone in a white coat is a serious scientist.
Further, some warn we may one day be able to incubate such foetuses outside the body, as described so memorably in Aldous Huxley's dystopian classic, Brave New World. Work to develop such "artificial wombs" is already under way. So is artificial reproduction on the horizon?
"I have no doubt there are people fantasizing about creating a baby with no humans involved," says Thomas Murray, president of the Hastings Center, a bioethics think tank in Garrison, New York.
"I am sure there are people intrigued by that prospect, though I'm not one of them. It is never too early to start thinking about the moral implications. It's amazing how quickly things develop and stun us," he said.
Those in doubt should pay a visit to the laboratory of Hung-Ching Liu, an embryologist at Cornell University in New York.
In 2002, Liu stunned the world of reproductive medicine by claiming to have recreated a woman's womb, using uterine cells grown on a biodegradable scaffold bathed in a broth of hormones and nutrients.
When Liu placed fertilized human embryos created during IVF treatment inside, they nestled into the wall of the womb and began to attach themselves to the endometrial cells that make up the lining -- just as in the early stages of pregnancy. Liu stopped the experiments after a week because regulations prevent human embryos being developed much further.
No such restrictions apply to animals and, in unpublished work, Liu says she has now grown mouse foetuses in her artificial womb for 17 of their 21-day terms. This is equivalent to about 31 weeks in humans, at which point babies have been viable for more than a month and can routinely be nurtured to normal development if born prematurely.
Just as with the human embryos, the tiny bundles of mouse cells nestled into the artificial womb lining and began to attach themselves. Liu watched as blood vessels formed, then miniature placentas and, eventually, the amniotic sac -- an embryo's personal protective bubble.
flaws
Liu says: "Normally people don't grow mouse embryos beyond 10 days. This goes way beyond that and forms a mouse shape housed inside a little bubble. It was wonderful. We were really amazed."
But, peering inside, Liu could see that something had gone wrong. "The foetuses were not healthy. We could see the mouse inside but it was severely deformed."
Liu repeated the experiment more than 150 times. Not all the embryos developed, but for those that did, the story was the same. By 17 days it was clear that the foetuses were abnormal, so she pulled them out. "They were like a stillborn baby, just sitting there, doing nothing. I don't think they were alive."
When Liu cut them free from their amniotic sacs, the mice were dead. She thinks that the problem lies in the animals' blood vessels, which do not form properly and so fail to circulate the required nutrients.
"What other factors it needs to develop into a normal baby is still unknown. We're only getting something that we think is close to the truth," Liu said.
Others are working at the other end of gestation, with equally startling results. A team at Temple University in Philadelphia, led by Thomas Shaffer, has developed breathable fluids that allow sheep delivered at half term to survive outside their mothers. And scientists in the laboratory of the late Yoshinori Kuwabara in Tokyo have used tanks of synthetic amniotic fluid to incubate late-stage goat foetuses taken from pregnant animals for several weeks.
Some have speculated that the two ends of this research will eventually converge -- allowing a two-cell embryo to develop into a living, breathing baby, entirely under laboratory lights.
Scott Gelfand, director of the Ethics Center at Oklahoma State University in Tulsa, was so concerned that he gathered experts together in 2002 for a conference titled The end of natural motherhood: The artificial womb and designer babies.
Murray, who attended the conference, says that while discussing the issue is easy, making it a reality is very, very difficult.
"I think it is crucial for us to work out where to invest our moral anxiety and I think that artificial wombs are not there yet," Murray said.
"They are much more complicated than people think and not a neat lab trick like squeezing out the nucleus from a cell and putting a new one in its place," he said.
Although the ever-dependable Raelian cult say they have developed a version called a Babytron to incubate their clones, no reliable scientist believes that we are anywhere close to a working artificial womb capable of replacing a woman.
Embryos being made from synthetic eggs and sperm, however, is a different story. The artificial eggs that prompted the errant newspaper's headline were prepared by a team led by Antonin Bukovsky at the University of Tennessee Graduate School of Medicine in Knoxville. Bukovsky says his technique could provide a potentially limitless supply of eggs -- a scarce resource in fertility treatment and stem cell research.
His claims have yet to be tested, and scientists have questioned why such groundbreaking results appeared in the little-known journal Reproductive Biology and Endocrinology, edited by Bukovsky. But it is clear in which direction research in the field is headed.
"It is not ridiculous to say this moves us towards the point where we can do completely artificial reproduction," says Josephine Johnston, also at the Hastings Center.
"But if you want a healthy baby, there are lots of easier ways and things people would rather do. It's a bit like the whole debate in IVF and how we could design babies, but the fact is that most people don't want to use IVF. They do it because they are desperate," she said.
cautionary tale
As John Eppig, a developmental biologist at the Jackson laboratory in Bar Harbor, Maine, puts it: "I'm sure bioethicists are already thinking about this, but even if the ability to do it comes along, I don't think it is going to replace the current method used to make babies in most homes."
Like Liu, Eppig has been experimenting with mice, and his results also tell a cautionary tale.
In 1996, Eppig succeeded in growing mouse eggs in his laboratory. He started with ovaries from newborn animals, cultured them and dissected out precursors of eggs, called oocytes, and associated cells. After careful nurturing, many of the resulting eggs began to grow when fertilized, but the 190 early-stage embryos transferred into female mice produced just one live pup. Eggbert, the first mouse born from a lab-cultured egg, was far from normal. He suffered from obesity and neurological problems.
Since then, Eppig's team has worked to improve the culture medium used to grow the eggs, and in 2002 it reported the birth of 59 apparently healthy mice. Others are working to produce synthetic sperm and eggs from less obvious sources: the ubiquitous stem cells.
In 2003, Hans Schoeler and Karin Huebner at the University of Pennsylvania's School of Veterinary Medicine, said they had produced eggs from stem cells extracted from mouse embryos. Others, notably Toshiaki Noce at the Mitsubishi Kagaku Institute of Life Sciences in Tokyo, have tried to repeat the trick with sperm, though it is proving more difficult.
Synthetic eggs and sperm made from stem cells raise new ethical questions, mostly over parenthood. Schoeler and Huebner's results suggest that eggs can be made even from male cells -- potentially allowing a gay male couple to produce children through sexual reproduction. Contrary to some reports, the same is not true for lesbian couples.
"To make a sperm you need a Y chromosome," explains George Daley, a stem cell biologist at the Children's Hospital in Boston. "There's been all kind of speculation about whether you could make sperm from female cells. You can't."
other issues
Daley adds his voice to the chorus insisting no reputable scientist is involved in this research because they think it could be used for reproduction.
"There are other issues that are more valuable to study, such as the development of the germ lineage, which has enormous implications for biology, fertility, the development of diseases and congenital defects. You can imagine all levels of bizarre scenarios but I think we need to stay focused on fundamental questions of medical importance," he said.
Besides, he says, sperm and eggs generated from stem cells in a laboratory will probably not develop properly.
"There are lots of reasons to think they are restricted or abnormal in some way and may not be able to support full development. This is a long, long way from reproduction in a dish," he said.
"Ethicists do need to be thinking about this and they do need to be thinking about it now. But actual applications are really not on the immediate horizon," Eppig said.
Were significant advances to be made along the road of artificial reproduction and gestation, the issues would clearly be significant.
"The issue with artificial wombs might not be so much in taking a foetus to term, but using them to save very premature babies," says Richard Ashcroft, a medical ethicist at Imperial College, London.
If hospitals could use such wombs to keep babies alive that would otherwise be too premature to survive, it could well have implications for abortion law, he adds.
But as with all technological advances, new techniques are not guaranteed a market. How many couples would want to see their baby grow in an artificial womb unless there was no alternative? And as Ashcroft adds, for the vast majority of women, the importance of going through the birth process cannot be overstated.
Artificial sperm and eggs arguably raise more profound issues. Knowing that genetic material came not from living humans but from synthetic gametes grown in a lab would reinforce the distinction between parents as genetic donors and those who raise the child.
For now, the sheer difficulty of perfecting the techniques necessary for fully artificial reproduction means the ethical issues are little more than talking points, Eppig said.
"All of these things are very, very hard to do. They are interesting mind games to discuss over a couple of beers after work," he said.
"While we're going to learn a lot about development from these studies, making embryos for animals and people is a long, long way off. How long? Don't hold your breath," he said.

Somehow, US intelligence identified “the Houthis’ top missile guy” and pinpointed his exact location. At 1348 hours (Washington time), March 15, President Trump’s national security advisor Mike Waltz texted, “positive ID of him walking into his girlfriend’s building.” The unsuspecting Romeo entered. High above, the drone monitoring the building registered a flash. When the smoke cleared, Mr. Waltz texted, “…And it’s now collapsed.” RIP. The star-crossed “top missile guy” had been target number one in the now uproarious US Navy bombing campaign on that Sunday against the Yemeni rebels who have been holding the Red Sea hostage since October 19,
China on Tuesday, April Fool’s Day, began two-day joint-force military exercises around Taiwan, painting them as a “severe warning and forceful containment against Taiwan independence.” However, the exercises have again proven the country increasingly showcasing its military muscles to be a true “troublemaker.” Without prior notice, the Chinese People’s Liberation Army’s (PLA) Eastern Theater Command launched large-scale exercises codenamed “Strait Thunder-2025A,” deploying aircraft, drones and naval vessels including the Shandong aircraft carrier, as well as armed militia in the air and waters around Taiwan. The PLA claimed the military exercises were practice for precision strikes and a blockade to “close
Taiwan on Monday celebrated Freedom of Speech Day. The commemoration is not an international day, and was first established in Tainan by President William Lai (賴清德) in 2012, when he was mayor of that city. The day was elevated to a national holiday in 2016 by then-president Tsai Ing-wen (蔡英文). Lai chose April 7, because it marks the anniversary of the death of democracy advocate Deng Nan-jung (鄭南榕), who started Freedom Era Weekly to promote freedom of expression. Thirty-six years ago, a warrant for Deng’s arrest had been issued after he refused to appear in court to answer charges of
Days ago, foreign media reported that Chinese People’s Liberation Army (PLA) Eastern Theater Command Director Lin Xiangyang (林向陽) is suspected to have disappeared under suspicious circumstances. The Eastern Theater Command is the core military department responsible for operations against Taiwan — the purging of its director, if true, would be a major blow to the morale of the Chinese military and the success of its training. On Tuesday morning — April Fool’s Day — the Chinese Communist Party’s (CCP) Eastern Theater Command suddenly announced the launch of joint military exercises in the air and maritime spaces surrounding Taiwan. The exercises